Reaction Measurements
Research work has concentrated on assessing and reducing uncertainties in the H2/O2 chemical model. We have worked closely with Prof. H. Pitsch and Dr. V. Terrapon by providing a highly reliable H2/O2 reaction mechanism for implementation in both low and high fidelity computations. Work has focused on addressing the major sources of uncertainty involved in the implementation of the H2/O2 chemical model.
We have made improved rate measurements for several H2/O2 reactions, including H + O2 -> OH + O, using shock tube/laser absorption methods. The reaction H + O2 -> OH + O plays a critical role in all aspects of hydrogen and hydrocarbon combustion; uncertainties in the rate coefficient of this reaction translate directly into uncertainties in ignition delay time and flame speed.
The rate coefficient of the reaction H + O2 -> OH + O was determined using tunable diode laser absorption of H2O near 2.5 mm behind reflected shock waves over the temperature range of 1100 to 1530 K. Excellent agreement between this study and that of Masten et al. (1990) was found in the overlapping temperature range.
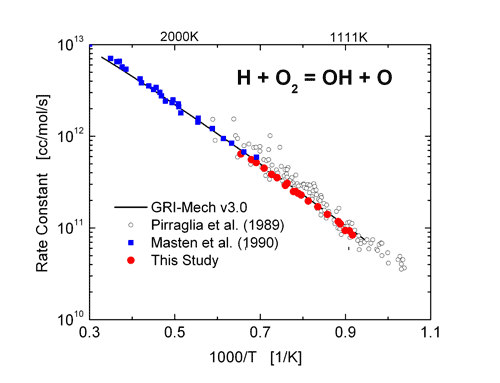
Arrhenius plot of the reaction rate of H + O2 = OH + O. Excellent agreement is seen with the higher temperature data of Masten et al. and significantly reduced scatter is seen when compared to the work of Pirraglia et al.
Mechanism Development
We have developed an improved H2/O2 reaction mechanism that incorporates recent reaction rate determinations in shock tubes from our laboratory. These experiments used UV and IR laser absorption to monitor species time-histories and have resulted in improved high-temperature rate constants for the following reactions:
H + O2 = OH + O (1)
H2O2 + M = 2OH + M (2)
OH + H2O2 = HO2 + H2O (3)
O2 + H2O = OH + HO2 (4)
The updated mechanism also takes advantage of the results of other recent rate coefficient studies, and incorporates the most current thermochemical data for OH and HO2. The mechanism is tested (and its performance compared to that of other H2/O2 mechanisms) against recently reported OH and H2O concentration time-histories in various H2/O2 systems, such as H2 oxidation, H2O2 decomposition, and shock-heated H2O/O2 mixtures. In addition, the mechanism is validated against a wide range of standard H2/O2 kinetic targets, including ignition delay times, flow reactor species time-histories, laminar flame speeds, and burner-stabilized flame structures. This validation indicates that the updated mechanism should perform reliably over a wide range of reactant concentrations, stoichiometries, pressures, and temperatures from 950 to greater than 3000 K.